Batterylife Activator
Review date: 21 March 2005.Last modified 03-Dec-2011.
Batterylife AG make the Batterylife Activator, a device which, they say, makes lithium ion batteries work better. Longer life, faster charging.
Well, calling the Activator a "device" might be overstating the case a bit. The product in question is, apparently, a "special ceramic foil", but to the untrained eye, it's a sticker.
A pretty fancy sticker...
...with lumpy stuff and little sparkly bits. But basically just a sticker.
Stickers that're alleged to do remarkable things to electronic devices do not have a very good reputation - see the end of this letters column for a commonly seen kind.
And then there are the phone-radiation-blocking stickers. I was most recently spammed by the lovely people I'll link from these words:
fraud rip-off scam spam scum lies
...(to do them a PageRank anti-favour, as opposed to just nofollow-ing 'em). There are plenty of others out there.
The claim that a sticker on the back of a mobile phone body does something to radiation coming out of the front of the phone antenna is a common one; if, of course, these things really blocked radiation, then the phone wouldn't work any more.
But hey - we live in the age of GPS, five-dollar hand-held lasers, 3-CCD hard disk camcorders and the F650 Super Truck and International CXT.
A sticker that makes batteries work better isn't, arguably, any less inherently plausible than cow Tasers, Lego automatic transmissions, the No-Sew No-Crochet User-Friendly Willie Warmer, Doom 3 on the Voodoo 2 or the Airbus Super Transporter.
The Activator is supposed to offer "up to 30% longer talk time, up to 30% longer standby time, up to 40% shorter recharging time, and up to 30% longer battery lifetime". As long as all of those "up to" parts aren't taking advantage of the old retailer's "up to 90% off!" dodge ("up to 90%" includes "0%"), the price is not excessive. You can buy Activators here in Australia now, though the where-to-buy page is a bit dead-endy at the moment; you're apparently talking about $AU30 for the mobile phone version. There's a larger laptop-battery version as well.
[The above batterylife.com.au links are to archived versions of the pages; Batterylife apparently went out of business a while ago, and batterylife.com.au is now owned by an unrelated company.]
How it works
Batterylife say that the Activator rejuvenates LiI batteries by counteracting the effects of metal particles floating around in the electrolyte.
They say the electrodes expand and contract as the battery's cycled, and bits of the electrodes break off in the progress, messing up the electrolyte and causing lower capacity and longer charge time.
I was kinda under the impression that LiI aging was caused by cell oxidation, and that old LiI cells generally took less time to charge, in proportion to the capacity they've lost. But hey, what do I know.
Anyway, the Activator's supposed to undo this damage by, ah, ionising the battery. There's apparently "up to 35 times" the normal ion density in the area around the Activator.
Now, I've heard these sorts of claims before. Not about a battery fixing gadget; the last such things that crossed my path (these devices - from a company that's now apparently very much defunct) were meant to reduce allegedly harmful cell phone radiation.
You stuck the poker-chip-sized device to your phone near the antenna, it supposedly passively produced lots of ions just like the Activator's meant to do, and those ions, uh, did something good. The explanation got kind of fuzzy around there.
Never mind what the ions were meant to do, though; there weren't any there in the first place. That's because there is no such thing as a passive ionising device. You need an energy input to make ions.
Ionisation is the addition or removal of electrons, to or from atoms or molecules or other ions. Electrons have a negative charge. Add one or more and you get a negative ion. Strip one or more away and you get a positive ion. Get back to the neutral, uncharged state and you've got a plain atom or molecule again, not an ion any more.
You could argue that the phone gadget got its energy from the phone's radiation and the Activator gets its energy from the battery somehow, but if that were the case, it'd be easy to detect the effect. Usually, you get ions in air from electrostatic effects (that's how regular home ionisers work - a big negative charge on their needles) or strong radiation. Alpha rays, in particular (that's how smoke detectors work). Gamma rays. X-rays. Positrons, neutrons, or electrons at speed. Fortunately, the Activator does not, I think, generate hard radiation.
Cell-phone radiation is not powerful enough to generate any ions. This is not one of those statistical things where one-hundredth of the power just makes one-hundredth as many ions; it's an energy-level thing, where you have to have more than energy X or nothing happens, just as someone not strong enough to push a car over a given hill will never be able to do it, even if he tries for a million years (I am, for the purposes of this simile, disregarding the concepts of muscle-building and erosion).
Falling water can generate negative ions, as well; some water droplets give up electrons to the air. This is still an energy-consuming process, though; something had to put the water up there before it could fall.
If the Batterylife Activator generates ions, it needs to consume energy to do it.
Also, even if it does generate ions - so what? You can spray all the ions you like on a battery, and you won't charge it. You can have a big ion cakewalk right through the middle of Tiananmen Square and it won't make a lick of difference, unless those ions are somehow participating in the electrochemical reactions inside the battery in such a way as to make it work better.
Never mind working better or worse - how the heck are the ions supposed to get in there in the first place? Is the battery casing being electrostatically charged all the way through? That wouldn't help, anyway - the cells inside have their own metal cases. The only way the ions could actually get from the sticker to the cells would be via some "quantum" flapdoodle or other.
The whole idea's ridiculous. It's like putting a sticker on the outside of your petrol tank and expecting it to alter the composition of the fuel.
(And yes, people have tried that too.)
My opinion on this subject is, of course, trumped by the opinions of people who've done fancy scientific tests of the Batterylife material and, apparently, reported that it performs as advertised.
Batterylife say that their product's been "evaluated, documented and certified" by "several prestigious institutions and universities" - including Osaka University, Kobe University and Japanese mobile comms giant NTT Docomo. They don't say exactly who at these institutions did this, or provide any documents for download; I e-mailed the universities and the local Batterylife rep about this a few days ago, but I haven't received any replies.
[I'm now giving this page a little going-over, fixing old broken links and such, more than three years after it first went up. I have still not heard from anybody about any real tests of the Activator. And it's not too likely that I ever will, seeing as Batterylife went broke rather a while ago now.]
The Activator has also, however, apparently been tested by TÜV Rheinland Group (a real, serious test and certification outfit), and Batterylife make that test report available for download (Zipped English PDF available for download from my server here, now that batterylife.de has disappeared). Those results look very positive.
(Batterylife left that report online for ages, although they didn't seem to, um, entirely stand behind it any more.)
So, the plot thickens. Either serious educational institutions and other non-trivial outfits have verified the extraordinary claims for the Batterylife Activator, and the nonsensical description of its method of action is inaccurate (or I've badly misunderstood it)... or it's all a pack of lies.
Empirical testing, ahoy!
Trying it out
Batterylife Australia were kind enough to send me the smaller Activator for review. Whereupon I struck a problem.
The official review guide tells you that the effects of the Activator may not be seen until the battery's been cycled as many as ten times, you see. It recommends you set up a test phone in an area that's got a mobile signal, then leave it in standby until the battery's flat, then put the Activator on the battery, cycle the battery five to ten times by normal use of the phone, then repeat the leave-in-standby test.
(The abovementioned review guide was downloadable in German and English. Now that batterylife.de is stone dead, I've mirrored the zipped PDF file for posterity on my server, here.)
Modern cell phones commonly have standby times of a week or two, and many people go at least a few days between charges. That means the ten cycles could easily take a month, and maybe rather more. And the before and after tests will take however long the standby time is - probably at least another fortnight, maybe another month.
You could stop people from calling (or SMSing) the phone and messing up your tests by taking the SIM card out (the phone should still connect to the network then), but you can't actually control how often the phone will chat with a base station during that period, sending a "heartbeat" signal so the cell knows the phone is still within range and active.
The number of "heartbeats" ought to be pretty much the same for any test period, but unless you station an intern in the test room with a pair of cheap computer speakers and tell him to make a note every time he hears the distinctive sound of cellphone handshaking coming through the speakers, it's impossible to tell.
I wanted a more repeatable, and preferably also faster, way to test the Activator.
Fortunately, one suggested itself. The batteries for my digital camera are just the right size for the cellphone Activator. The instructions also say it's OK to cut down an Activator to fit smaller batteries; the only rule is that at least 80% of the battery's footprint should be covered by the sticker.
The BP-511 batteries my camera uses have a nominal 7.4 volt, 1100 milliamp-hour (mAh) rating. The Activator is meant to work only on batteries that're at least nine months old, so I rooted through my camera bag and found a couple of likely suspects.
The first was an off-brand battery that wasn't terribly old but, charging attempts revealed, was down to maybe a thirtieth of its original capacity; regular readers won't be surprised by that. I decided that nothing short of divine intervention was going to fix that one, and went instead for a genuine Canon battery that was older than the dead off-brand unit, but still had 70% of its original capacity left. I guess there is a difference, huh?
I established the battery's capacity not by using it in my camera, but by hooking it up to a standardised load. If you want to test a battery, that's the way to do it; hook a set resistance up across its terminals, and graph voltage over time. You can do the graphing with a $5 multimeter and a stopwatch, manually noting the voltage every minute or whatever, but I've got a fancy multimeter with a serial port on it. It lets me do the logging automatically on my PC, and then import the results into Excel and make a hideous graph.
My test resistance couldn't be too low, or I'd hurt the battery; it couldn't be too high, or I'd be old and wizened before the tests ended. I opted for a 12 ohm string of resistors.
For best results, lithium-ion batteries shouldn't be loaded to more than "1C". The C there stands for the capacity of the battery; the idea is that a 1000mAh-capacity battery shouldn't be asked to deliver more than 1000mA of current.
A freshly charged BP-511 can deliver about 7.8 volts into 12 ohms, which means the initial current is about 650 milliamps. That's about 0.6C if you take "C" to refer to the original 1100mAh capacity; it's still under 0.9C for a battery like this one that's down to 70% capacity.
As the battery empties, the output voltage and current will drop, giving a gentler and gentler discharge as time passes.
Discharging a whole multi-cell battery to zero volts, or close to it, is not a good idea. The cells won't have quite the same capacity, and the ones that run out of juice before their comrades will be "reversed" - charged backwards - by the last trickle of current coming out of the stronger cells. Cell reversal damages the weaker cells more, and so the problem snowballs every time you do a deep discharge.
"Smart" batteries, including many lithium ion models, deal with this problem by using internal electronics to disconnect the output terminals when the battery's state of charge drops below some set level. The voltage-versus-time discharge plot for these kinds of batteries looks like that of any other battery, except it drops vertically to zero at some point rather than sloping gently all the way down.
Lithium ion, however, delivers a nominal 3.7 volts per cell, so it's quite possible for various small devices, including mobile phones, to run from "batteries" that only contain a single cell. That makes it safe for them to be fully discharged, so they often just contain basic safeguards to stop the cell from indulging in lithium ion's favourite pastime, catching fire. A thermal fuse or circuit breaker of some kind is all they really need.
My BP-511 is a two-cell battery, with cutoff hardware, so it didn't matter if I left the discharge setup alone for hours; I just had to trim a lot of zeroes off the end of the log file when I came back.
LiI batteries are, by the way, pretty unlikely to display weird behaviour as a result of weird usage patterns. Nickel-cadmium and nickel-metal-hydride batteries can benefit from occasional deep cycling to deal with voltage depression, so putting a Mickey Mouse sticker on one and then cycling it a few times may give you the impression that the sticker's rejuvenated the battery.
LiI batteries aren't like that. They have no "memory" of any sort, and cycling them doesn't rejuvenate them in any way. They don't much care whether you leave them charged or discharged, and their self-discharge rate (the rate at which they lose their charge when they're just sitting there doing nothing) is low.
What did my test setup look like?
I'm glad you asked.
Resistors and multimeter probes screwed into nylon terminal strip, which is attached to workbench with NASA-spec Science Nails.
Multimeter not shown.
The steam and Stirling engines are not part of the apparatus.
I tack-soldered on a couple of fly leads to make it easy to hook the battery up. With the leads tacked on the ends of the contacts, I could still get the battery into the charger. The Activator didn't get in the way either, after I made a couple of little cuts to allow access to the terminals and the clips on the side.
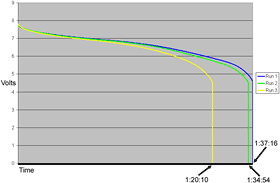
Before I took the above pictures, I ran three tests without the Activator on the battery. The battery sat on the charger overnight between the first and second tests, then had a regular-length charge before the third.
Each time, the open circuit voltage of the battery was about 8.4V, dropping to around 7.8 under load.
(Click the graph for a bigger version that you can, you know, read. I've marked the times when the pack ran out of juice and got cut off by its protection hardware.)
As you can see, each successive test showed less capacity, which is pretty much what you can expect from a tired old LiI pack that's being discharged and recharged over and over. Leave it to recuperate for a while and it'll deliver much the same performance each time.
But all that was about to change, according to the nice people from Batterylife. On went the sticker, and it was Cycle Time.
Over the next few days, I gave the battery ten cycles, as recommended. Some were back-to-back; some had a few hours or a whole night between them. To preserve the surprise, I didn't monitor the battery's performance during the cycling. I just charged it, hooked it up to the resistors to dump the charge, then charged it again, while my fancy multimeter slumbered nearby.
Before the final run, which I was going to monitor to see what effect the Activator-assisted cycling had had, I let the battery rest for a few hours after its last cycle discharge. Then I left it on the charger for a couple of hours past the point where the charge was officially complete, to make sure it was as topped off as it was going to get.
The moment of truth arrived, and...
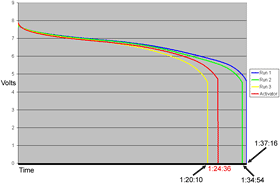
Well, so much for that.
The Activated battery scored better than it did on the third of its un-Activated test runs, but I'd given it some time to rest and then a definitely complete charge, which it might not quite have had for the third run before I put the Activator sticker on it.
The Activated result is just what you'd expect from an old LiI pack that's ten cycles further into its life than it was before. It's slowly on the way out, as are we all, and no sticker's going to help it.
Oh, and the thing didn't charge any faster, either. Batterylife promised that it would.
Shorter charge time for LiI doesn't actually make a lot of sense. Well, at least not as a good thing.
LiI and lithium polymer batteries are charged in a very definite way, you see. At a constant current, set by the charger, until a particular tightly defined voltage per cell is reached, then constant voltage until the charge current falls off to near zero.
If some gizmo gives a LiI battery more capacity, the constant-current portion of the charge will pretty much have to take longer. You're putting water into a bigger bucket at the same rate.
But LiI batteries certainly can charge quickly - if they're knackered. A battery with very little capacity left will still be chargeable, but it'll reach its peak cell voltage very quickly - possibly in a few minutes, rather than the few hours it probably takes to charge a new battery.
A product that does this to your battery is unlikely to please you.
Dissenting opinions
It is my considered opinion, based on the above, that the Batterylife Activator not only has an incoherent and ridiculous claimed means of action, but also commits the cardinal sin for any gadget: It doesn't bloody work. At all.
Batterylife have, however, scored positive reviews from people all over the planet. A Lithuanian magazine (PDF) liked it (I think...), as did these people in Switzerland, these people in France (who, uh, sell Activators, but maybe that's just because they're so convinced they're great...), these people in Portugal, these people in Germany, and these people and these people in the UK. And PC Games Hardware magazine in Germany (PDF), and someone in Bulgaria (PDF), and this free magazine (screencap), which seems to give complimentary reviews to pretty much everything it sees, but which may do that because it only reviews really good stuff. Who knows.
Then again, Spode saw "no useful effects", and Bit-Tech "couldn't possibly recommend you buy the Activator at any price". So I'm not alone.
Assuming, for the sake of argument, that the Activator doesn't work, how come so many people say that it does?
Well, the cynical explanation is that they're all on the take, and/or idiots.
The more charitable one is that while some of them may not be saying what they really believe, or may not be too good at figuring out what they really believe, most of them just didn't do good science.
If you don't properly control your test situation - if, for instance, you just walk around with an Activatored phone and see if you reckon you have to charge it less often - then you can easily get the wrong end of the stick. Leave science by the wayside, and you're pretty much certain to end up believing weird things.
Further fun
After I put this review up, the local Batterylife people had, you'll be surprised to learn, some complaints.
They said the battery tested wasn't on the compatibility list printed on the product packaging - true enough, but the Activator's supposed to work on any lithium-ion battery.
The specific problem with this one was, apparently, that since it was an "external heavy-duty battery" (which it wasn't; it goes inside various Canon consumer and pro cameras), it had a "heavily shielded casing" (which it doesn't; it's a thin plastic shell like that of various other batteries), which "interferes with the proper operation of the BatteryLife product" (which, I remind you, is also meant to work with laptop batteries, which, like my BP-511, contain cylindrical cells in a roughly rectangular casing).
But I'm nothing if not willing to waste yet more precious minutes of the rapidly-dwindling time remaining in my life to give purveyors of questionable products every opportunity to redeem themselves. So I peeled the Activator off the battery, ripped the plastic lid right off the pack, and re-stuck the Activator to the bare cells inside.
I'm happy to report that Batterylife now say that the Activator "should be placed as closely as possible to the actual cell" (note once more - the way I had the thing installed before was exactly the same as the best way you could possibly install the larger laptop version on a laptop battery).
OK, though. You can't get any closer than this, folks!
The lidless pack still charged just fine. I ran it through yet another ten cycles, and measured its performance for the last three.
I could spend time making up another pretty graph of the results, but the curve shape was the same; it's the end-time that mattered. And wouldn't you know it, that was the same too. 1:02:19 for run number eight (which was the last in a sequence of three back-to-back cycles, so the battery was feeling rather tired), 1:18:46 for run nine, and 1:18:20 for run ten (for the last two runs, I gave the pack a rest before recharging it).
No change. No improvement. A bit worse, actually, because of the extra cycles for this poor old battery. And, thus, no case to answer.
Mind you, Batterylife Australia's news page also now contains the following quote from the German parent company:
"the reason for this bad result... is Shock-discharge... to make the test shorter which won't work. No phone in the world will be "shock-discharged" with normal use. So this test is not representative at all, respectively... with this kind of test a result is impossible."
Oh, really?
They make Activators for laptop batteries, which certainly can be discharged in about an hour and a half. You generally have to buy a big "desktop replacement" machine that uses a cheap non-"mobile" CPU to suck the batteries dry that fast, but those are quite popular these days. There are plenty of higher-powered laptops that get little more than an hour of battery life - especially from the cheaper six-cell battery packs - if you're playing a 3D game or otherwise working the system hard.
But maybe the laptop Activators have different trans-spectral Jeffries tubes, I hear you say. What about phones?
Well, video-capable "3G" mobile phones also have this kind of battery life, if you're one of the hip young things who uses the video feature. Here's one. Here's one that only manages an hour. It's not hard to find others.
If you discharge a normal lithium-ion battery in less than an hour, you are indeed probably mistreating it, as I mention above.
So I didn't do that.
Thank you, and goodnight.
After this, I presume the Batterylife people will come up with some other ad-hoc objections. To save them time, I have prepared the following quick cut-and-paste list.
- Peeling the Activator off something and sticking it back on again misaligns its subquantum regions and renders it useless.
- Peeling the Activator off a battery will permanently ruin that battery, on account of proactive synergy.
- The Activator doesn't work when you stick it on a curved thing, because its theory of operation requires a flat space-time.
- The Activator doesn't work if the plastic shell of the battery is too thick, but it also doesn't work if the shell's too thin, or not there at all. Quantum tunneling, don't you know.
- Dan is a stupidhead.
I'm ignoring them from now on, unless they can point me to those actual independent published results that they say prove that Activators work.
Overall
Maybe the Batterylife Activator works. Maybe The Wine Clip and the EMPower Modulator do, too, and my reviews of all three products constitute vile calumnies.
But I think I've given this thing as fair a trial as anyone (except, perhaps, those university and NTT Docomo people that Batterylife AG can't quite identify...), and it's proved to be an utter waste of money.
I will, furthermore, go out on a very long limb and claim that, in my humble opinion, BatMax Battery Life Boosters don't work either. I base this on the fact that while more fancily described, the Booster seems to be the exact same thing as the Activator.
(Astoundingly, I am not alone in this opinion.)
One day, perhaps, we'll see a miracle sticker that performs as advertised.
I'm not holding my breath, though.
My review Batterylife Activator was kindly provided by Batterylife
Australia.
Batterylife AG seem to be
out of business now, though, and batterylife.com.au is now owned by an unrelated
company.